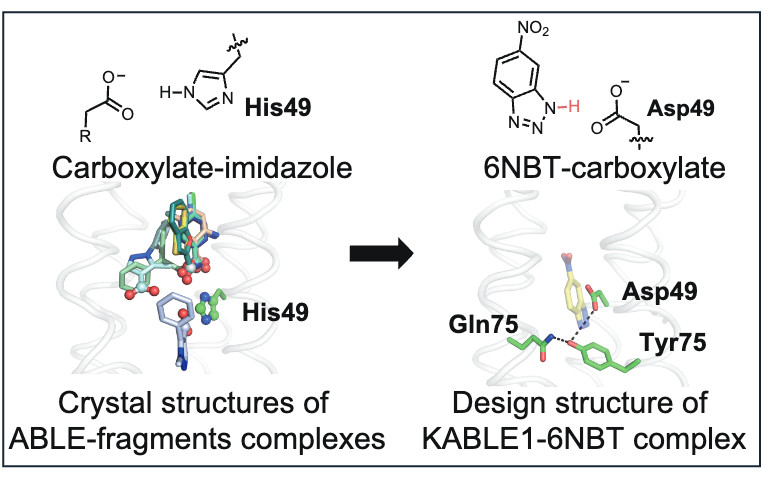
Next, we took inspiration from more polar fragments binding lower in the original binding site and designed KABLE (Kemp eliminase ALBE). With a few additional mutations from a single round of screening it has a catalytic efficiency (600,000 M⁻¹s⁻¹) on par with the natural enzymes.
Comments
- Mutagenesis confirmed Asp49 as the essential active site residue
- Disrupting key orienting H-bonds also eliminated activity
- kcat/KM for analogs scaled with the electron-withdrawing nature of leaving groups
We think this mimics how evolution explores generalist binding sites to generate new functions.
Link to preprint: https://www.biorxiv.org/content/10.1101/2025.01.30.635804v1
We welcome feedback!