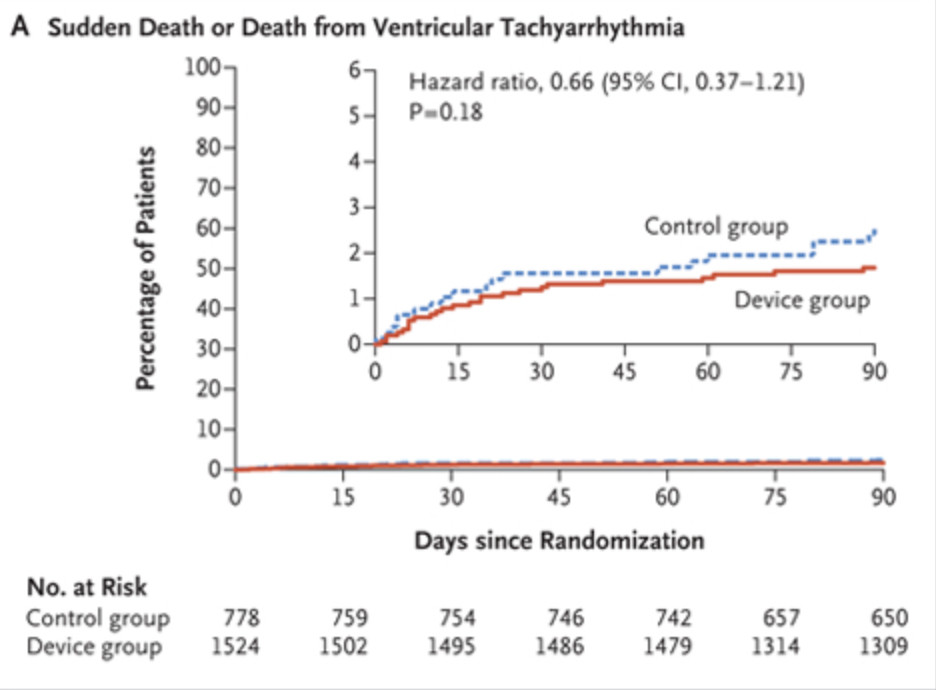
When patients get fitted for a Zoll Lifevest for low EF, is the evidence supporting this, the VEST Trial (https://pmc.ncbi.nlm.nih.gov/articles/PMC6276371/)?
The one that is only positive on post-hoc, Zoll-sponsored, analysis?
https://pmc.ncbi.nlm.nih.gov/articles/PMC9374026/
The one that is only positive on post-hoc, Zoll-sponsored, analysis?
https://pmc.ncbi.nlm.nih.gov/articles/PMC9374026/
Comments
https://www.acc.org/Latest-in-Cardiology/Articles/2020/10/01/01/42/Focus-on-EP-The-Wearable-Cardioverter-Defibrillator-A-Life-Vest-of-Controversy
Bottom line: I wish I invented the LifeVest.
In many cases limited evidence of benefit, in some even evidence of harm!
I’m still surprised the failure of the Nellix AAA stent hasn’t garnered more widespread attention. 👇
At a minimum proper post-market registries for new devices are a must!
https://www.sciencedirect.com/science/article/pii/S1078588420309977
I rare rx it, and have never had someone had an appropriate therapy, but did once have my guy show up holding the button, saying "this thing won't shut up" when he was in slow VT at like 110
This is an interesting meta. VEST is the only RCT
https://www.jacc.org/doi/10.1016/j.jacep.2018.11.011#appsec1
Said otherwise, almost never. Other cases where you use it?
Tangentially related: the evidence for primary prevention ICD in NICM is poor or absent.
See: DANISH
https://www.nejm.org/doi/full/10.1056/NEJMoa1608029
And in the super rare case of an event there’s no wiggle room for suing (that I know of)
Recently a patient with LQTS and multiple appropriate shocks (most recently in June). Lead impedance too high and cannot guarantee effective defib. But patient getting chemo for acute leukemia. Disabled ICD and added wearable defibrillator.
Is there a cardioEPSky community?